Controlling Slag Defects, Insoluble Buildup in Melting and Holding Furnaces
During the past 30 years, the melting methods and associated molten metal-handling systems used by the U.S. foundry industry have changed significantly. Further, the quality of metallic scrap and other iron-unit feed stocks has steadily deteriorated. The result: slag generation and slag-related melting problems have become widespread issues in recent years. A search of the foundry technical literature about slag control and buildup from the past 30 years finds only a handful of articles.
As most iron foundries use silica-based refractories in their coreless melting, they have been reluctant to add fluxes. Fluxes are used extensively throughout the integrated steel industry because they reduce the melting point of difficult-to-melt impurities and alloys for separating metal and gangue during smelting.
In the foundry industry, there has been a historical reluctance to use fluxes. Improper use of fluxes can rapidly erode refractory furnace linings, especially if potent fluxes are used. Typically, operator error can cause problems with fluxes. "If a little works well, then a lot should work better," doesn't necessarily apply. Doubling or tripling the amount of recommended flux additions can result in reduced lining life, especially with highly reactive fluxes. Meanwhile, foundries have been convinced that using any flux at all will reduce refractory life significantly, often without having proper knowledge of the flux chemistry or potency.
Newly engineered fluxes can extend the refractory life of coreless induction furnaces by up to 60% and more. Other benefits include increased service life of pouring vessels, and these fluxes can significantly reduce the incidence of slag and/or dross inclusions.
The formation of slag in the melting of ferrous metals in the foundry industry is an inevitable process. The compositions of slags vary with the process used and the type of iron or steel being melted. The cleanliness of the metallic charge, often including sand-encrusted gates and risers from the casting process or rust-and dirt-encrusted scrap, significantly affects the type of slag formed during melting.
Additional oxides or nonmetallic compounds also can be formed when liquid metal is treated with materials to remove impurities (deoxidation) or to change the chemistry of the system (inoculation and nodulizing.) Because these oxides and nonmetallics are not soluble in iron, they float in the liquid metal as an emulsion. This emulsion of slag particles remains stable if the molten iron is continuously agitated, as in the case of the magnetic stirring inherent to induction melting. Until the nonmetallic particle size increases to the point where buoyancy effects countervail the stirring action, the particles will remain suspended.
When flotation effects become great enough, nonmetallics rise to the surface of the molten metal and agglomerate as a slag. Using fluxes accelerates this process. Failure to remove these emulsified slag particles may lead to costly slag inclusions or dross in castings.
Iron casting defects originating from slag or dross carry-over is often a leading cause of unacceptable gray and ductile iron castings. Several iron foundries and consultants have stated that slag or dross defects ranks at or near the top of the list of problems raised in daily scrap evaluations.
Many times these defects are referred to as dirt, dross, slag or other foreign substances that are embedded in or just below the cast surface. Because these castings are either gray or ductile iron, weld repair is usually out of the question. When these defects show up in the cleaning room, after costly in-foundry processing has been applied, the negative effect is more dramatic.
Slags and dross from electric melting, if not totally removed at the melting furnace, will be transferred into the pouring ladles. Because the walls of the pouring ladle are much cooler than the furnace refractory lining, slag buildup is inevitable. The task of continually maintaining clean pouring ladles requires significant labor, and failure to do so may result in costly casting scrap from slag inclusions.
Fluxes prevent slags and other insolubles from freezing on the cooler refractory surfaces. Using a flux usually ensures floatation of the emulsified oxides and reduces the melting point of the slag to below the lowest temperature encountered in the melting furnace and associated liquid metal handling system. Fluxes also affect the surface tension of slags. Further, fluxes allow for the coalescence of low melting point slag droplets that otherwise may become emulsified in the liquid metal bath of high frequency induction furnaces.
Viscous non-metallic slags can negatively affect coreless, channel, and pressure-pour furnaces, causing slag formations on the furnace and/or inductor walls. The adhesions and/or build-up interfere with melting, thereby decreasing furnace efficiency. It is not unusual to have two inches or more of slag buildup occur on the walls of coreless, channel furnaces, pressure-pour furnaces, or ladles within the first several hours of operation.
Proprietary fluxes based on a blend of alkaline oxides have been developed for use in electric melting furnaces, pressure-pour furnaces, ladles, and certain ductile iron treatments to solve these problems. These new fluxes provide excellent cleansing action comparable to fluorspar, however, it is not aggressive toward furnace linings and is environmentally friendly (i.e., no fluoride emissions.) On of these fluxes — Redux — is available in 45-gram tablets as well as pre-weighed 1-lb. powder packs for ease of use. Adding 1-2 lbs. of flux per ton of molten metal is sufficient to cleanse the metal, remove slag, prevent buildup formation on furnace walls, on channel furnace / pressure pour inductors.
Slag/Dross Inclusion Elimination Case Histories
Foundry A melts both gray and ductile iron in several large channel and coreless melting furnaces. Foundry A melts roughly 160 tons per day of both gray and ductile iron, producing a broad range of castings weighing from 50 lbs to several tons.
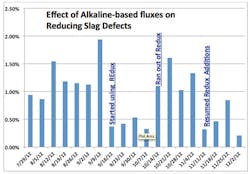
Prior to using Redux, total casting scrap due to slag had been running close to 1.26% on average, in spite of using filters on almost every casting. Switching alternately from ceramic foam to pressed, then to extruded filters showed no reduction in scrap rates associated with “dross or slag”.
In mid-September 2012, Foundry A started by adding just 1 lb. of Redux per ton of metal to every five-ton ladle, and quickly found the incidence of defects associated with slag dropped to roughly 0.43%, a 65% reduction. In mid-October, Foundry A ran out of flux, and almost immediately casting scrap increased dramatically to pre-flux levels. Upon re-ordering flux and adding it at the same 5 lb. of flux to each and every five-ton ladle, scrap rates associated with slag plummeted again. The reduction in slag-related casting scrap that Foundry A achieved after five months of using Redux is shown in Figure 1.
Foundry A continues to add flux to every ladle on a daily basis, and has realized an estimated annual savings of over $500,000 per year. During calendar year 2013, overall slag defects averaged 0.38% of total production and Foundry A is continuing to make improvements in its melting and metal handling systems to reduce slag-related defects even further.
Foundry B is an international ductile iron foundry that was seeking a method to extend the service life of its ladles. The foundry also was seeking a method to keep the tundish pocket clean and reduce the use of magnesium ferrosilicon by improving magnesium recoveries.
• After 8-16 hours of production, a 1-ton tundish treatment ladle showed significant buildup in the treatment pocket and side walls. Buildup on the ladle sidewall was typically 2 in. or more, and more important the tundish pocket was almost entirely engulfed with slag, leaving little room for the magnesium ferrosilicon treatment alloy. The tundish ladle was lined with a 85% alumina, low-moisture castable with backup insulation.
• After five treatments with Redux briquettes, the tundish pocket volume was restored and the ladle sidewalls were free of slag.
Prior to using Redux, service life was 8-16 hours of production before the ladle was removed from service for pocket cleaning. Adding just 1 lb. (0.45 kg) of flux to each ladle (placed on top of the cover steel) cleaned the ladle and extended the service life of the ladle to 72 hours.
The benefits of using Redux with every ductile treatment at Foundry B were:
• Improved metal cleanliness and ladle refractory cleanliness;
• Reduced labor costs via reduction of chipping and patching of ladle pocket and walls;
• Increased production via longer service life of treatment ladle as the pocket maintained its volume;
• Reduced material costs via less refractory patching, extended campaigns for the ladle refractories;
• Improved consistent magnesium recoveries and reduced magnesium ferrosilicon consumption.
In addition to being used to keep ladles clean and free of slag buildup, incorporating 1-1.5 lbs. of flux has improved the inductor life of pressure-pour furnaces and coreless induction furnaces around the world. Alkaline-based fluxes can extend coreless furnace refractory life significantly by preventing slag buildup. By keeping furnace volumes constant, melting efficiency and melting productivity is vastly improved.
David C. Williams is v.p. - Technology and Rod Naro is president and CEO of ASI International Ltd.
About the Author
Dr. R. L. (Rod) Naro and D. C. Williams
Rod Naro is president and CEO, and David C. Williams is v.p. - Technology of ASI International Ltd. Visit www.asi-alloys.com