Latest from Ask the Expert
Q: Recently I was testing a new, solvent-based refractory coating but after diluting with my solvent, the Baumé level increased, rather than decreased as I had expected. What happened?A: When it comes to refractory coatings, not all formulations are made alike. In fact, they can be very different, not only in refractory blend, suspension agents, clays, binders, etc., but they also can vary greatly in the composition of the “carriers” … or, the solvent package used to establish “flow” for the product. The same distinction applies to the solvent that you might use to dilute your coating to a specific density or viscosity level before use.
Most refractory coating products are delivered as heavy slurries that require a certain amount of dilution with solvent — in this particular example, an alcohol solvent. Alcohol solvents come in different grades or purity levels, and in foundry coating products the most common types have 99% and 91% purity. The 99% purity alcohols are very low in moisture content and are more flammable, but also dry faster.
The 91% solvent typically contains 9% water (or possibly a mix of water and other impurities), which can slow down drying and have negative effects on a solvent coating, depending on the type of solvent used to produce that coating. If lower-purity solvent is used to dilute a coating made with higher-purity solvent down to a working viscosity/Baumé level, the high level of water in that solvent can significantly affect the suspension/clay package. The net effect usually is an increase in the viscosity of the coating instead of the desired outcome, a reduction in viscosity. You end up with a very thick coating with much less refractory material than you may have expected based on the dilution method.
Therefore, it is critical to understand what type of solvent you are going to use to dilute your coating, and to work with your supplier to understand if it is in fact compatible with the product you are going to dilute.
The higher-purity, 99% isopropyl alcohol solvent can be used to dilute either a coating made with 99% IPA or 91% IPA, or one that may contain a certain amount of water in the formulation for various reasons (like reducing flames after light off or improving brush-ability.) However, it is not the case that 91% IPA solvent can be used to dilute a coating made with 99% IPA or has a very low water content. If this is done, you will find it very difficult or even impossible to dilute your coating to a reasonable viscosity/Baumé level for application purposes. In the end, it is always a good idea to consult with your supplier before starting dilutions of any kind, as he or she will understand the compatibility issues and also provide you with detailed guidance on dilution and application levels for that particular product.
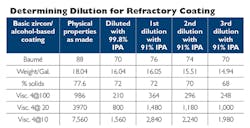
The table shown here provides an example of the potential outcome when using lower-purity alcohol solvents to dilute a coating that was produced using a higher-purity IPA. You will see in the first column the original properties for a solvent-based coating produced with 99%+ alcohol. In the second column you will see the properties/viscosities of this same coating when diluted down to a working Baumé of 70 degrees using a pure 99% alcohol. Reading from left to right, you’ll see the dramatic differences when you use 91% isopropyl alcohol to dilute the same coating.
Once you finally arrive at the same, targeted working Baumé of 70 degrees, you’ll see that your percent-solids and density are significantly lower than the desired levels. This could lead to casting-quality issues relating to an insufficient amount of refractory material applied to the core and/or mold. The lesson of all this is: know the formulation of the refractory coating you are starting with, and what solvent you are using to dilute it, as these details will influence the coating properties you are able to achieve — and the quality of the castings you are able to produce.
Join the Conversation. Email Your Questions for ASK Chemicals
Share your insights, opinions, and elaborate on the questions and the experts' answer(s). You must be logged in to the website in order to post your comments.