Understanding Contamination From Feeding Aids In Cast Steel
Feeding aids are intended to improve the performance of risers and prevent shrinkage in the casting, while optimizing yield. Feeding aids include insulating and exothermic riser sleeves and different types of hot toppings for open risers. The aids function to keep the risers liquid longer by reducing heat losses and/or providing additional heat in the case of exothermic materials.
Insulating feeding aids slow the loss of heat from the riser. To do so, they must have a lower thermal conductivity and specific heat than the surrounding mold. Insulating sleeves are typically a low-density refractory material, like aluminum-silicate fibers or low-density aluminum-silicate ceramic (LDASC) spheres1. These materials are chemically inert even at metalcasting temperatures and do not interact chemically with the liquid metal. In addition to their insulating properties, the sleeves also must have sufficient strength to withstand pressure applied during molding and from the head pressure of the metal. This requires some type of binder to make the sleeve relatively rigid and strong. Many different binder types may be used, but alkaline phenolic and phenolic urethane formulations are commonly used. These organic binders break down at casting temperatures and are a potential source of carbon and nitrogen.
Insulating hot-topping can be in powder / granular form, or they may be solid2. They may contain organic materials like sawdust or rice hulls that burn away, but leave a powdery ash with good insulating properties. They also can be refractory materials like expanded perlite. Generally, the solid hot toppings and the insulating sleeves are made from the same types of materials. The organic and solid hot toppings are potential sources of carbon and nitrogen from the breakdown of the organic materials and binders.
Exothermic sleeves contain some type of "fuel" that ignites and provides additional heat when exposed to the liquid steel: Thermite is the most common type of fuel.
Thermite contains powdered aluminum, iron oxide, or other metal oxides, and some type of initiator like cryolite (Na3AlF6) or nitrates. Thermite may be mixed with sand or with an insulating refractory. The sand exothermic sleeves typically have higher amounts of thermite to produce additional heat, but have poor insulating properties after the thermite is consumed. Insulating exothermic sleeves usually have lower amounts of thermite and will provide some insulation after ignition is complete. They rely on the combination of heat input from the thermite and moderate insulating properties after ignition. Also, the exothermic sleeves will have the same types of binder used in the insulating sleeves. Exothermic sleeves have the potential to contribute aluminum, oxygen, carbon and nitrogen to steel in the riser.
Exothermic hot topping...
Exothermic hot topping typically contains a combination of thermite and insulating material. They have the same possible contaminants as exothermic sleeves. However, liquid iron also can be produced from the thermite reaction, and can fall and be absorbed by the metal in the riser, providing a more direct route of contamination.
Carbon pickup in steel may occur from exposure to carbon-rich gas produced from the decomposition of organic materials, including binders. The amount of carbon pickup is controlled by a number of factors3. Binder types and amounts top the list. Phenolic urethane binders generally produce more carbon pickup because of the high carbon content and low oxygen content of the polymer. The greater the amount of binder the more pickup can occur. Sand additives like iron oxide can reduce carbon pickup somewhat by providing oxygen to the mold atmosphere. However, no previous testing has been done to show the effects of thermite additions that include iron oxide. Carbon pickup also is controlled by section thickness and cooling rates. Because risers are designed to stay hot longer than other portions of the casting, this could exacerbate any carbon pickup into the riser.
It appears that carbon pickup occurs primarily through carbon diffusion into and through the solid, similar to surface carburization in a heat-treating furnace. There appears to be little direct carbon pickup into the liquid steel. This means that there is little risk of direct carbon transport from the riser into casting during solidification, but that risers may have higher carbon levels when remelted.
Much has been written about nitrogen pickup and control in steel. Melting operations, transfer of molten metal, carbon raisers and ferroalloys, heat treatment, and welding are all mentioned as potential sources of nitrogen. However, less has been written about nitrogen pickup in the mold. It is well know that nitrogen pickup occurs in iron castings from the decomposition of organic binders. Shell, furan, and phenolic urethane binders all contain some nitrogen. The form of the nitrogen as hexamine in shell binder or isocyanates in other systems makes it particularly easy to breakdown to atomic nitrogen, which is very soluble in liquid metal. Given the higher temperatures and higher solubility for nitrogen in steel than in iron, some pickup is likely. Some additional nitrogen pickup also may occur after solidification by the same solid transport as seen with carbon. This would result in still higher nitrogen levels in the risers at remelt.
Aluminum is a normal constituent of many steel casting alloys. It is used as a final deoxidizer of the melt prior to pouring. Aluminum pickup has also been associated with the use of exothermic materials containing thermite4, with reported pickup levels of 0.03 to 0.10 for exothermic sleeves and levels of 0.09 to 0.13 for hot toppings. Some metalcasters avoid the use of exothermic sleeves because of concern over potential aluminum pickup5. Previous work by the authors6 showed that significant amounts of aluminum could be picked up in ductile iron from exothermic riser sleeves. This causes a loss of nodularity and a reversion to flake graphite. Levels as high as 0.71% aluminum were seen in the ductile iron in risers with highly exothermic sand sleeves. Subsequent work7 linked the high aluminum pickup to the cryolite initiator used in the sleeve formulation. Formulations that were free of fluorides showed significantly less aluminum pickup in ductile iron.
If aluminum and nitrogen are present in the steel in the riser in sufficient amounts, they can precipitate as an aluminum nitride (AlN) phase. This precipitation is controlled primarily by the levels of aluminum and nitrogen and by the cooling rates. The AlN is very brittle and precipitation at the grain boundaries can result in “rock-candy” fractures at very low loads8. Pickup of aluminum or nitrogen or both from a feeding aid could exacerbate the AlN formation.
Depending on the feeding pattern from the riser into the casting, AlN could feed from the riser into the casting. There could be additional concentrations of AlN if there is under-riser segregation caused by the breaker core on the riser. Breaker core material, opening size, and geometry can each be important in controlling segregation9. Also, research has been conducted to develop modeling capability to predict the formation of brittle AlN phases10.
Round 1 testing...
Round 1 testing — Given the known potential for carbon, nitrogen, and aluminum pickup from a variety of sources in liquid metal, it seemed simple to quantify the amount of pickup into steel risers and to determine the important variables controlling those amounts.
Initial testing was conducted to identify the amount of contamination from exothermic sleeves into the riser and casting on steel cube castings. Several different types of 2.5X3.75-in. riser sleeves were gathered and molded on 3-in. cube castings. These included exothermic fiber sleeves from two different suppliers, a sand exothermic, and two LDASC sleeves with different levels of exothermic material. B50 breaker cores were used on each sleeve. These were selected because previous testing had shown some degree of alloy segregation with this size breaker core.
The castings were poured with low-carbon steel at 1,620°C (2,950°F) and allowed to cool overnight in the molds. Following shakeout, the castings were sectioned to show shrinkage and the feeding pattern. Then, one half of the riser was ground and macro-etched to show areas with potential carbon pickup or alloy segregation.
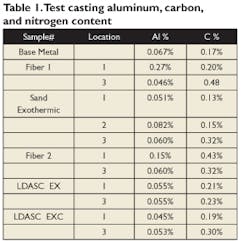
The risers also were analyzed for aluminum and carbon at select locations. Drillings were taken from different points on the riser, and analyzed. The base chemistry for the heat and riser analyzes are shown in Table 1.
The two fiber sleeves showed areas of elevated aluminum and carbon but these were not consistent throughout, making it impossible to determine if the higher levels resulted from pickup from the sleeves or were from alloy segregation in the riser, or both. It was decided to take a more systematic approach with tighter control on the sleeve composition and expanded test locations.
Round 2 testing...
Round 2 testing — The next round of tests focused only on LDASC sleeves with variations to the exothermic package. The commercially available composition normally used for small steel casting was set as the standard (sleeve 6) and the amount of thermite was cut to two-thirds (sleeve 2), increased to one-and-a-half (sleeve 3), and the cryolite initiator was changed from cryolite to a nitrate initiator with the standard (sleeve 4) and one-and-one-half thermite packages (sleeve 5). An insulating sleeve with no thermite at all was included, too, as a control (sleeve 1). Table 2 shows the various compositions tested.
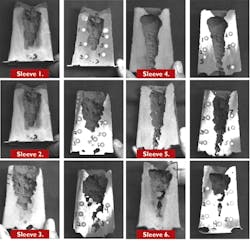
The 2.5X3.75-in. sleeves were again placed on 3-in. cube castings and poured at 1,620°C with low-carbon steel. As with the previous tests, the risers were sectioned, ground, and macro-etched to show areas of potential alloy segregation. Then, the sections were analyzed for carbon, aluminum, and nitrogen at multiple locations using an emissions spectrometer. The riser sections are shown in Figure 1 and all of the spectrographic data is shown in Table 3.
None of the risers showed a consistent increase in carbon content, although all showed evidence of surface carburization in a layer less than 1 mm thick. This was apparently the effect of solid diffusion after the riser had solidified. Some of the sleeves showed isolated spots with elevated aluminum, but only sleeve 3 with 1.5 the thermite showed slightly higher aluminum levels throughout. However, sleeve 5 that also had 1.5 thermite but with the nitrate rather than cryolite initiator did not show elevated aluminum. All of the risers showed somewhat higher nitrogen levels with some isolated spots showing much higher levels. All of the sleeves were molded using phenolic urethane binder at the same levels. Because the binder was the primary potential source of nitrogen and the total binder levels were the same, variations in the nitrogen in the sleeves cannot be easily explained.
Round 3 testing...
Round 3 testing — To investigate further the effects of the total amount of thermite and the initiator in the sleeves, another set of tests was conducted. This time, sleeves were made using sand rather than LDASC. The same percentage of thermite was used as the standard EX LDASC sleeve, but because of the higher density of the sand the sleeves weighed about 2.5 times as much and contained about 2.5 times as much thermite. Since sleeve 5 from the previous tests had the elevated thermite, but used nitrate rather than cryolite as the initiator, sand sleeves with nitrate also were included in the testing. Standard LDASC EX and insulating sleeves were included as controls. Castings and risers were produced in duplicate, too.
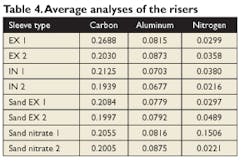
The risers were tested again at multiple (typically, seven) locations, but rather than showing all the data, it is summarized in Table 4 showing the average carbon, aluminum, and nitrogen levels in the sleeves.
Some of the average values appeared to be elevated, particularly for the nitrogen levels. However, a review of all the values showed that one or two of the seven readings were much elevated, either from an erroneous reading or from potential segregation, and that most of the values were in the lower range. Note that all of the aluminum levels were very similar and that even increasing the total aluminum in the sleeve by 2.5 seemed to have very little effect.
Effects of riser size — During the course of the initial trials, relatively small, insertable sleeves were used, for speed and convenience. However, the effects of riser size were also debated. A smaller, sleeved riser would have a smaller modulus or volume-to-surface area ratio. This should mean that the sleeve could have a more significant effect because of the ratio of the surface area of the sleeve to the volume of the riser. However, it was also argued that larger risers would take longer to solidify with more time for aluminum or other contaminants to become dissolved.
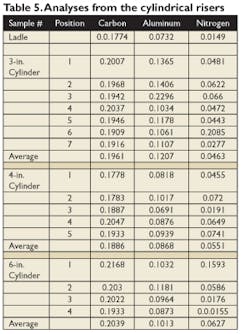
Cylindrical sleeved risers with 3-, 4-, and 6-inch diameters were poured with the same low-carbon steel used in other testing. The LDASC sleeves all had the same EX formulation used in the other tests. As before, the sleeves were sectioned and subjected to spectrographic analysis at a number of locations. The results are shown in Table 5.
All of the risers seemed to show a slight increase in aluminum, and nitrogen levels were both higher and more erratic. However, there did not seem to be any trends associated with riser size.
Hot topping trials — Logic suggested that the “worst case” opportunity for contamination into a riser would be with a hot topping. The material would be in direct contact with the molten metal in the riser and any exothermic materials could potentially contribute contaminated molten iron directly into the riser.
Trials were conducted with open, 3.375-in. cylindrical risers on 4-in. cube castings. The risers were not sleeved and were surrounded by the same no-bake mold sand as the cube. No breaker cores were used. A control with no hot topping also was used. Test castings included two thicknesses of insulating material with no thermite, 0.5 in. of 100% thermite, 0.5 in. of a 50:50 blend of thermite and insulating material, and 0.5 in. of a 25:75 blend of thermite and insulating material. After pouring with low-carbon steel at 1,620°C, measured amounts of the different hot topping materials were immediately applied to the open risers to ensure the correct thickness. Following solidification and cooling, the risers were sectioned and the top 0.5 in. was cut from the riser and analyzed for aluminum.
None of the tops of the risers showed any significant increase in aluminum levels.
Conclusions...
Conclusions — Initially, we expected to see a certain amount of aluminum contamination and to see some correlation between the amount of exothermic materials in the feeding aid and an impact from the type of initiator. In one sense it was disappointing that we could not reliably reproduce the aluminum pickup reported by others. It is very difficult to “fix” or improve something that you cannot accurately measure.
However, the fact that we could not produce significant amounts of aluminum pickup even with very elevated amounts of thermite in the formulations strongly suggests that the problem may not be as serious as it is perceived among steel casting foundries. The initial tests with fiber sleeves suggested some pickup, but little was seen with any of the LDASC sleeves. At this point we certainly cannot say that there is no aluminum contamination, but it must be recognized that it is very difficult to “prove” the negative.
The isolated aluminum pickup that was indicated in the tests seems to be related to alloy segregation or other local phenomenon rather than to overall increases in aluminum from the sleeves or hot topping. This should provide some assurance that exothermic sleeves and hot topping can continue to be used as an effective tool for controlling shrinkage, improving yield, and reducing cost. It also may provide a warning that improper riser or breaker core design can cause segregation and potential under-riser cracking, whether exothermic or insulating risers are used.
REFERENCES
1. Aufderheide, R.C., Showman, R.E., Twardowska, H, “New Developments in Riser Sleeve Technology,” AFS Transactions 1998, Paper 98-07.
2. Aufderheide, R.C., Mathias, J.M., Waters, K., “New Hot Topping Techniques Improve Riser Feeding Consistency,” AFS Transaction 2007, Paper 07-098
3. Showman, R.E., Lowe, K.E., “Controlling Carbon Pickup in Steel Castings,” AFS Transactions 2010, Paper 10-005.
4. “Contamination of Steel Castings From Riser Sleeve and Topping Materials with Reference to Under- Riser Cracking,” Research Report No. 91, Steel Founders’ Society of America
5. “Insulating or Exothermic? Steel Casters State Their Cases,” Blair, M., Monroe, R., Modern Casting, May 2011.
6. Showman, R.E., Lute, C.A., Aufderheide, R.C., “Exothermic Riser Sleeves Can Cause Flake Graphite in Ductile Iron,” AFS Transactions 2001, Paper 01- 086.
7. Aufderheide, R.C., Showman, R.E., Close, J., Zins, E.J., “Eliminating Fish-Eye Defects in Ductile Castings,” AFS Transactions 2002, Paper 02-047.
8. Dutcher, D.E., “Understanding ‘Rock Candy’ Fracture in Steel Castings,” Modern Casting, February 1999.
9. Aufderheide, R.C., Showman, R.E., Jain, N., “Breaker Core Optimization,” AFS Transactions 2010, Paper 10-017.
10. Monroe, C.A., Huff, R.K., “Prediction of Aluminum Nitride Embrittlement in Heavy Section Steel Castings,” AFS Transactions 2010, Paper 10-094.