Iron oxide threatens all iron-melting processes. In cupola melting, much greater amounts of iron oxide are produced during the normal melt cycle than are produced in electric furnace melting. This makes iron oxide’s effect much more pronounced in cupola melting. In EF melting, 20%-30% oxidation loss is the extreme of losses encountered. In cupola melting, in the worst-case scenario, the oxidation losses approach 65%. Carbon oxidation losses remain unknown to this day.
When a cupola is first started, blast air “burns-in” the coke bed. During this phase, no iron oxide is produced because no iron is melted. The blast air simply preheats the coke, preparing for the upcoming melting campaign.
Once metallic charging and melting commences, blast air contacts the molten metal droplets as they descend through the oxygen-rich layer immediately in front and above the cupola tuyeres. This oxygen-rich high-temperature zone (5,000°F) in the cupola causes iron-oxide formation on the droplet’s surface and vaporization of a portion of the iron oxide formed. The FeO gas spreads throughout the cupola, leading to FeO contamination of the entire cupola.
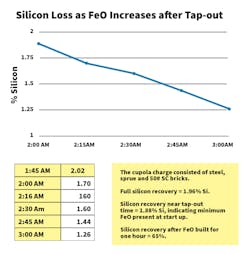
Iron oxide causes significant chemistry losses, both carbon and silicon. The graph below shows silicon loss as melting progresses in a 35-tph cupola. Silicon loss finally reaches steady-state when FeO exiting the cupola contained in slag equals the FeO being produced by blast air contact with the molten metal.Iron oxide present in the tuyere raceways produces carbon-oxidation loss. There have no meaningful measurements of the carbon loss in the raceway due to the ever-changing dynamic nature of the cupola melt zone.
Mastermelt DeOX counters iron-oxide formation in the raceways: The elusive carbon loss does not need to be determined. DeOX injection simply eliminates the loss, making further investigation moot.
DeOX is the only material available that eliminates iron oxide in the tuyere raceways. As stated previously, iron oxide formation cannot be stopped but DeOX instantly chemically reduces it to inert by-products. With iron oxide and its effects neutralized, free-oxygen atom levels in the molten iron bath decrease near to oxygen’s inert level of activity, effectively de-oxidizing the cupola melting operation
First contact. The mechanism of the oxidation loss reactions is iron oxide contacting the molten iron droplet’s surface, immediately adding free-oxygen atoms to the molten iron. Those oxygen atoms seek out other elemental atoms within the iron’s matrix and combine (oxidation process) in accordance with the Laws of Thermodynamics. High temperature (as in the tuyere raceways) favors carbon loss, and low temperature (as in the upper melt zone) favors silicon loss.
The amount of iron oxide formed controls the amount of the loss. Once free-oxygen atoms enter molten iron, there is nothing available to iron melting personnel to stop the oxidation reaction. The only option available to them is to somehow cut off the oxygen atom supply.
In steel melting, operators add aluminum (0.03% Al) to quickly combine with free-oxygen atoms but this option is not available to iron melting personnel due to pin-holing sensitivity in cast iron, promoted by elevated levels of aluminum.
The volume of iron oxide produced in the tuyere raceways is controlled in part but, significantly, this is a consequence of the blast air’s humidity. High humidity makes cupola melting very difficult.
Most coke suppliers will comment on how cupola-melting personnel always claim coke quality deteriorates during the hot-summer months. Obviously, coke quality has not deteriorated.
The fact is more iron oxide is produced during melting and the increased iron-oxide level causes increased carbon and silicon losses. A typical scenario in the high-humidity periods: increase coke rate; add or increase pig iron; reduce steel in the metallic charge; enter complaint with coke suppliers.
Up until now, nothing was available to reduce iron-oxide formation in the tuyere raceways. Mastermelt’s DeOX is available now to counter – not stop – iron-oxide formation.
Natural course. Iron oxide forms naturally – by the Laws of Thermodynamics. Mastermelt’s DeOX, however counters iron-oxide formation by catalytically reducing it into inert by-products as it forms.
Cupola melting encounters carbon oxidation losses in the tuyere raceways, and encounters carbon pick-up directly below the raceways from the incandescent, graphitized coke. Carbon is lost and then gained, and the overall effect is a net increase in carbon. Cupola operators refer to the overall carbon pickup as “carbon from the coke bed.”
In the past, the carbon loss occurring in the tuyere raceways has gone un-treated. Two schools of thought existed with cupola operators: either the carbon loss was unknown, or nothing could be done about it, so no concern was assigned to it.
The common practice used to increase molten iron’s carbon content was to raise coke rates – the famous “coke bump”.
Carbon increase occurs from coke that has transitioned through the 5,000°F temperature range directly above tuyere level. This high temperature causes the amorphous coke to convert to graphitic-carbon atom structural arrangements. Once the coke has been graphitized and descends below the oxygen-rich blast air region, carbon dissolution into the descending iron droplets readily occurs.
Carbon pick-up can vary significantly, but it is highly dependent on the metal temperature. The volume of coke below tuyere level remains constant so addition of “coke bumps” or other temperature control means to produce increased carbon appears to occur simply due to metal temperature increases. Carbon’s dissolution rate into molten iron is significantly affected by metal temperature.
Cupola operating temperature is a critical operating parameter that requires much investigation and review. Temperature of molten iron exiting the cupola becomes the main focus for melt performance improvement once iron oxide’s presence within the cupola is countered.
Consider the following cupola operating parameters, which affect a cupola’s molten iron temperature:
1) Uniform blast-air distribution between tuyeres;
2) Proper blast-air penetration away from the cupola’s shell;
3) Streamline blast-air flow;
4) Minimize retained slag level – proper metal dam height;
5) Proper-size coke;
6) Proper-size metallic charge materials;
7) Reduced cement bonded charge materials;
8) Proper amount of limestone; and
9) Inject oxygen uniformly in tuyeres; use special injection nozzles.
Numerous benefits result when cupola operating improvements are instituted:
1) Improved refractory service life throughout the cupola;
2) Straight-line metal chemistry control – with no oxidation losses;
3) Eliminate pig iron and other special metallic charge materials;
4) Eliminate chemistry declines over hold periods; 5) Provide instant chemistry recovery after cupola down periods;
6) Reduced coke rates;
7) Reduced slag volume produced in melting cycle;
8) Substantial cost savings;
9) Improved metal quality; and
10) Precise management of metal quality.
Take control. The first and foremost step to improving cupola operation is to gain control of iron oxide formation throughout the melt process. Once iron oxide contamination of the cupola is resolved, coke rates can be reduced to the 6-7% level.
The reduced coke rates produce dramatic energy efficiency improvement and corresponding reductions in greenhouse gas emissions. Slag volume produced is cut in half. Monetary savings are significant – millions of dollars saved annually.
Controlling iron oxide completely changes cupola operations: No slag buildup occurs in the front box. No tap hole erosion occurs. No wide variations in metal chemistry occur.
Each of the points listed above will be discussed further in coming articles, and these and many more operating points will be discussed in our upcoming cupola operating workshop.
A simple change like a two-inch reduction in metal dam height can increase metal temperature by 60°F. a four-inch reduction in tuyere height increases metal temp 75°F. Obtaining uniform blast air distribution eliminates isolated wear areas within the cupola, reduces coke rates, reduces chemistry variations, and increases metal temperature.
Then consider this: limestone additions vary dramatically from cupola to cupola. Reducing limestone increases metal temperature, decreases chemistry loss, increases metal temperature, and enables easier slag handling in the front box. Cupolas today operate at 10 to 45% limestone additions based on coke weight. Nothing different happens from one cupola to the next, although each melt department superintendent insists, “the limestone used is the limestone needed.”
This is a falsehood that must be discussed. Limestone adds nothing to metal quality, and it is added to counter coke ash’s silica content. Ten-percent limestone works at one cupola, it should work at all cupolas.
Melt operators must consider the effect of coke size on combustion energy produced. The coke salesman promoting 10%, tiny-sized coke substitution for normal-sized coke is misleading: Coke combusting to form CO2 liberates nearly four times the heat released by coke combusted to form CO.
CO formation is favored by the large carbon-surface area found with small coke. Large coke burns hotter: Adding 10% tiny coke substitution should be stopped. Anyone considering small-size coke should consider reducing the overall coke rate by the amount of the proposed tiny coke addition rate.
No minimum coke-rate is required to produce a certain melting result. Coke rate determines metal temperature only after the cupola is de-oxidized with Mastermelt DeOX Metal Treatment. Seven-percent coke rate is being used now in operating cupolas in the U.S., indicating that all cupolas can operate at that level.
Cupolas operating at low coke rates today do so due to no significant carbon level increase being required during the melt cycle. DeOX Metal Treatment removes this restriction, providing increased carbon levels without requiring increased coke rates.
DeOX Metal Treatment technology maximizes cupola operating performance.
Ron Beyerstedt is the president of Mastermelt LLC. Contact him at [email protected]
This is the second in a series of reports examining cupola design, cupola melting practice, and cupola technology solutions. See Maximizing Cupola Performance, FM&T March 2021.